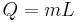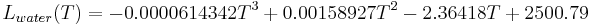Latent heat
The expression latent heat refers to the amount of energy released or absorbed by a chemical substance during a change of state that occurs without changing its temperature, meaning a phase transition such as the melting of ice or the boiling of water.[1][2] The term was introduced around 1750 by Joseph Black as derived from the Latin latere, to lie hidden. In meteorology, latent heat flux is the flux of heat from the Earth's surface to the atmosphere that is associated with evaporation or transpiration of water at the surface and subsequent condensation of water vapor in the troposphere. It is an important component of Earth's surface energy budget. Latent heat flux is commonly measured with the Bowen ratio technique, or by eddy covariance.
Contents |
Usage
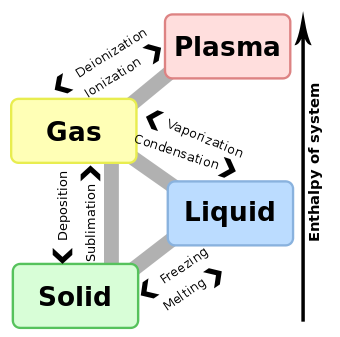
Two of the more common forms of latent heat (or enthalpies or energies) encountered are latent heat of fusion (melting) and latent heat of vaporization (boiling). These names describe the direction of energy flow when changing from one phase to the next: solid → liquid → gas.
In both cases, the change is endothermic, meaning that the system absorbs energy on going from solid to liquid to gas. The change is exothermic (the process releases energy) for the opposite direction. For example, in the atmosphere, when a molecule of water evaporates from the surface of any body of water, energy is transported by the water molecule into a lower temperature air parcel that contains less water vapor than its surroundings. Because energy is needed to overcome the molecular forces of attraction between water particles, the process of transition from a parcel of water to a parcel of vapor requires the input of energy causing a drop in temperature in its surroundings. If the water vapor condenses back to a liquid or solid phase onto a surface, the latent energy absorbed during evaporation is released as sensible heat onto the surface. The large value of the enthalpy of condensation of water vapor is the reason that steam is a far more effective heating medium than boiling water, and is more hazardous.
Specific latent heat
The specific latent heat is the amount of energy required to convert 1 kg (or 1 lb) of a substance from solid to liquid (or vice-versa) without a change in the temperature of the surroundings – all absorbed energy goes into the phase change – is known as the specific latent heat of fusion. Likewise, the amount of energy required to convert 1 kg (or 1 lb) of a substance from liquid to gas (or vice-versa) without a change in the external temperature is known as the specific latent heat of vaporization for that substance. Tables of values for the two specific latent heats are published; values for some common substances are given below.
The latent heat for a different mass of the substance can be calculated using the equation:
where:
- Q is the amount of energy released or absorbed during the change of phase of the substance (in kJ or in BTU),
- m is the mass of the substance (in kg or in lb), and
- L is the specific latent heat for a particular substance (kJ-kgm−1 or in BTU-lbm−1); substituted as Lf to represent as the specific latent heat of fusion, Lv as specific latent heat of vaporization.
In other words, specific latent heat is found when energy is divided by mass, or as represented as 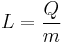
Table of latent heats
The following table shows the latent heats and change of phase temperatures of some common fluids and gases.
| Substance | Latent Heat Fusion kJ/kg |
Melting Point °C |
Latent Heat Vaporization kJ/kg |
Boiling Point °C |
|---|---|---|---|---|
| Alcohol, ethyl | 108 | −114 | 855 | 78.3 |
| Ammonia | 339 | −75 | 1369 | −33.34 |
| Carbon dioxide | 184 | −78 | 574 | −57 |
| Helium | 21 | −268.93 | ||
| Hydrogen(2) | 58 | −259 | 455 | −253 |
| Lead[3] | 24.5 | 327.5 | 871 | 1750 |
| Nitrogen | 25.7 | −210 | 200 | −196 |
| Oxygen | 13.9 | −219 | 213 | −183 |
| R134a | −101 | 215.9 | −26.6 | |
| Toluene | −93 | 351 | 110.6 | |
| Turpentine | 293 | |||
| Water | 334 | 0 | 2260 (at 100oC) | 100 |
Latent heat for water
To calculate the latent heat of condensation in water in the temperature range from −40 °C to 40 °C the following empirical cubic function can be used:
with a determination coefficient of 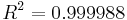 , where
, where 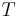 is in °C.
is in °C.
See also
- Bowen ratio
- Sublimation (physics)
- Specific heat capacity
- Enthalpy of fusion
- Enthalpy of vaporization
References
- ↑ Perrot, Pierre (1998). A to Z of Thermodynamics. Oxford University Press. ISBN 0-19-856552-6.
- ↑ Clark, John, O.E. (2004). The Essential Dictionary of Science. Barnes & Noble Books. ISBN 0-7607-4616-8.
- ↑ Textbook: Young and Geller College Physics, 8e, Pearson Education
- ↑ Cubic fit to Table 2.1,p.16, Textbook: R.R.Rogers & M.K. Yau, A Short Course in Cloud Physics, 3e,(1989), Pergamon press
|
|||||||||||||||||||||||||